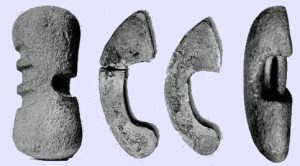
Halteres (dumbbells) were used to assist in jumping: some examples survive in museums:


The earliest dumbbells for strength training are shown in a woodcut from Hieronymus Mercurialis’ De Arte Gymnastica Aput Ancientes, first published in 1569 in Venice, Italy. One of the most important aspects of Mercurialis’ text was the shape of the handweights. No longer curved like the ancient halteres above, the dumbbells pictured in Mercurialis’ text resemble two conical pyramids stuck together by their heads.
On display in Dr Tom’s Museum is a pair of dumbbells in this exact fashion, discovered in France, which are in all likelihood not dating from Ancient Greek times
(Ancient Greece was a civilization belonging to a period of Greek history that lasted from the Archaic period of the 8th to 6th centuries BC to the end of antiquity (ca. 600 AD).




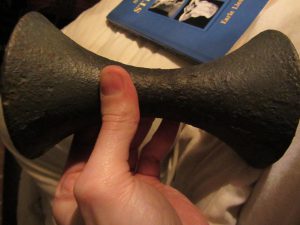
although it would be wonderful to think they are such survivors! Experts like David Horne (UK) believe these may have been fabricated by some enterprising would-be fitness-type after publication of Mercurialis’ text. Certainly they are very early, and it is astonishing that they have survived together. Short of metallurgical analysis (which needs to be done as soon as practically possible) it is probably impossible to establish anything further about these fascinating bells…
Interesting text showing how they were used:

Some features for readers: here is a thin layer of what looks like green paint over them, which may have preserved the base metal material – which seems to be iron, but this is an unknown. They weigh 3.5kg each.
The Tabula Plumb (Plummets) shown in the Mercurialis illustration in the link, are made of stone or lead – and the halteres above are all made of stone.
This text signposted to me by Jarrett Hulse (USA) is also informative;
‘The following figures, which more readily explain this, were copied from brasses in the possession of the Prince of Ferrara, about the middle of the sixteenth century. In the engraving may be seen the rollers for the chest and ribs, as mentioned by Galen, and the abdominal region greatly inflated as is wont to take place when the breath is forcibly detained. But the reason for these figures appearing with shaven heads, with hair alone upon the very summit, and carrying
plummets or dumb-bells hanging from the left arm, is, we think, both because they were slaves, and because they were skilled in using the dumb-bells. For we read that many nations shaved the heads of their slaves ; just as they shaved the entire heads of the Dacas, a German nation conquered by Trajan, except so much’ as could cover the crown’


So, to date these dumbbells: Tom says: ‘basically just been sitting there, and I just haven’t had the time to get any analysis done – also I have an idea that it might be pretty expensive – but just haven’t had the space to do anything with them – so it’s basically ‘on hold’… wish I could say more, but there it is. I don’t think analysis means taking them apart – it would be in the realms of a tiny sample – but you are right.. it would cause destruction.. there is another proposed method which is interesting: the dumbbells appear to ‘painted’ with something, which is wearing off and exposing iron, which has then corroded.. so…
Radiocarbon Dating of Rust
A second interesting area concerns the use of rust for dating. If rust can be dated reliably, it opens up a large number of possibilities for dating iron artifacts. Investigators will not need to cut into valuable artifacts for clean metal, but will be able to use surface corrosion products. This potentially opens the way for dating precious samples such as the iron plate found in the Great Pyramid at Gizeh,10,11 now at the British Museum. It may also be possible to date completely rusted artifacts, commonly found in waterlogged early Iron-Age sites in Europe and in underwater shipwrecks. Previous investigators had been careful to remove rust from iron prior to dating for fear that it adds contamination. A key issue though, is whether any of the original carbon remains within the matrix of rust and other corrosion products. If not, rust and similar materials are clearly of no interest for radiocarbon dating and should probably be removed since, at best, they can do no good. However, if original carbon is present, the corrosion products themselves may be appropriate targets for dating, subject to solving the potential contamination problems.
Most of the carbon in iron-based materials is in the form of the orthorhombic, crystalline iron carbide (Fe3C) known as cementite. Morphologically, cementite appears either as spheroidized particles or as pearlite. For compositions exceeding the eutectoid level of about 0.8% carbon, the excess carbide often exists as massive plates of proeutectoid cementite. The thickness and sizes of all of these carbides can vary enormously, depending upon composition and heat-treatment history. For steels that have been quenched to form martensite (body-centered tetragonal structure), the carbon is essentially in solid solution in the iron up to the eutectoid composition, beyond which it too will usually be in the form of carbides.
Despite the complex range of possible amounts and morphologies of the cementite, the thermodynamic stability of iron carbide is significantly greater than that of iron. So, as iron rusts, the carbide phase will be more stable than the matrix and will remain behind. The question then becomes one of kinetics: How long will it take for the carbide to oxidize compared to the iron matrix? As long as the carbon remains in the rust, in whatever form, it will potentially be available for radiocarbon dating.
Although little appears to have been published on this subject, Knox12 reported the detection of iron carbide in the remaining oxide from a corroded 2,800-year-old Iranian steel dagger.
Optical metallography explicitly showed the presence of “unaltered” iron carbides in the rust from this object. Also present in the microstructure were pseudomorphs of carbides, which had been reduced by corrosion to carbonaceous regions that were “probably an intimate mixture of oxide and amorphous carbon.” More recently, Notis13 has succeeded in making carbon maps in rusted old steel samples using electronprobe microanalysis techniques at Lehigh University and has observed good carbon images in the microstructures.
The present authors and van der Merwe14 have recently completed a study in this area. This work provides some evidence for the reliability of dating corrosion products from artifacts that have rusted in the air, in the ground, and under water, although it does not prove that all such samples can be successfully dated. Nonetheless, iron samples that had completely rusted produced plausible radiocarbon dates, but issues of contamination and post-depositional carbon exchange must be thoroughly tested in a variety of field settings before rust dating can be considered a validated technique. The present authors’ results indicate, however, that in at least some circumstances, the original carbon from iron-based materials is retained in rust and can be cleanly extracted and dated (see Figure 1a and Figure a). What the study does show, then, is that there is no a priori reason why the method should not work on rust. The work suggests that accurate radiocarbon dates may be obtainable with minimal material and with minimal risk to artifacts.